In 1900 Max Planck was investigating how the radiation emitted from a black body was related to its temperature. He discovered that the energy radiating from a vibrating object was quantized. He found that the quantized energy was proportional to the frequency of vibration through a constant now known as Planck’s Constant. $$h = e/f$$
where \(e\) is the quantized energy, \(f\) is the frequency of the vibration, and \(h\) is Planck’s constant.
In 1905 Einstein proposed the photon theory of light. It was for this theory that Einstein was awarded the Nobel Prize in 1921. Using the quantization idea introduced by Planck five years earlier, Einstein proposed that the transfer of energy to and from light and other electromagnetic radiation took place in packages, or quanta, of size $$E = hf,$$ where \(E\) is the energy of the photon, \(f\) is the frequency of the radiation, and \(h\) is Planck’s constant.
In this Lab we are going to use Einstein’s theory and the light emitted from LED’s (Light-Emitting Diodes) to calculate Planck’s Constant. Light-emitting diodes are studied in depth in Physics 2130. This Lab offers a simplified explanation as to how they work.
A diode is a type of electronic component that allows current to flow in only one direction. A light-emitting diode (LED) is a diode that emits light when current flows through it. Inside the LED are two poles made of Gallium alloy crystals. The two poles are separated by a gap called a Band Gap. One pole is known as a P-type and the other is known as an N-type. The N-type has an excess of electrons and the P-type is missing electrons (the places where the electrons are missing are called “holes”). When provided with a large enough energy source connected across the two poles, electrons from the N-type become excited and jump the gap between the two poles and fill the electron holes of the P-type. As the electrons fill the holes, they fall back to their unexcited state, thus releasing energy in the form of photons. The color of the light emitted by the LED corresponds to the energy of the emitted photons and is determined by the amount of energy required for an electron to cross the Band Gap. One can change the effective size of the Band Gap by changing the alloy make up of the P and N poles. Thus one can change how much energy is required for an electron to jump the gap, ultimately determining the color of the light emitted by the LED. The larger the band gap is, the higher the energy is of the photon that the LED emits. So a small gap would emit a photon near the red end of the spectrumand a large gap would emit a photon near the blue end of the spectrum.
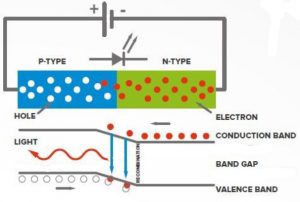
simplified sketch of an LED courtesy of Light National Direct, UK
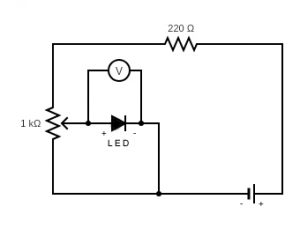
A diagram of the experimental circuit is shown above. By changing the resistance of the 1 kilo-ohm potentiometer, we can change the amount of potential (\(V\)) between the N and P poles of the LED. We want to achieve just enough potential to excite an electron on the N pole and cause it to travel across the Band Gap to the P pole where it will drop back down to its unexcited state and give up a photon of light. By measuring this potential (\(V\)) between the two poles, one can calculate the Kinetic Energy of the electron as it moves across the Gap, $$K.E._{\rm{max}}= q\cdot V$$where \(q\) is the fundamental charge of an electron and \(V\) is the potential (voltage drop) across the Band Gap. This \(K.E.\) is thus equal to the amount of energy of the photon that is emitted when the electron falls back to its unexcited state.
As you can see this follows the principle of Einstein’s theory of light. However there is one thing that we have overlooked. LED’s are not 100% efficient. Not all of the Batteries’ energy is transformed into light. Some of the Batteries’ energy is transformed into heat. To account for this we need to apply Conservation of Energy principles to Einstein’s Energy equation from above. We will introduce the Greek letter \(\phi\) as the term for this energy loss. So after applying C.O.E. to \(E = hf\) we get $$K.E._{\rm{max}} = hf – \phi$$ This is our Key Equation.


